|
Satish Lele lelebiodzl@gmail.com |
| Materials for Titration | |
|
|
Reagent: Dissolve 10 gram of KOH in 1 liter of distilled or de-ionized water (1% KOH solution).
Basic titration : In a smaller beaker, dissolve 1 ml of used cooking oil in 10 ml of pure isopropyl alcohol. Warm the beaker gently by standing it in hot water, stir until all the oil dissolves in the alcohol and the mixture turns clear. Add 2 drops of phenolphthalein solution to get end point.
Using a burette, add 1% KOH solution, drop by drop to the oil, alcohol, phenolphthalein solution, stirring all the time, until the solution stays pink (magenta) for 10 seconds. Note the number of milliliters of 1% KOH solution you used. The reading in milliliters (ml) gives you percentage of Free Fatty Acid content of used cooking oil.

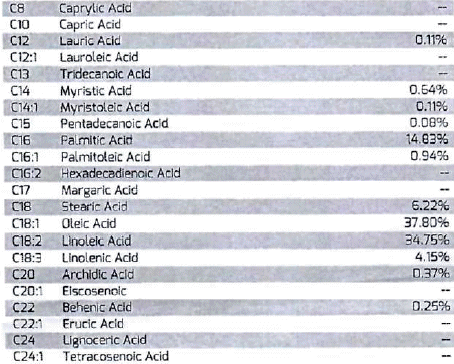
Typical Fatty acid Profile of such oil